
Article by George Haughn
The primary plant cell wall of land plants is an essential cellular structure needed for support, cell-cell adhesion, signaling and interaction with both the biotic and abiotic environment. It is composed of a complex, dynamic extracellular matrix consisting of a network of carbohydrate polymers (cellulose, hemicellulose and, pectin) and structural proteins. Wall composition can vary dramatically both throughout the life of individual cells and between different cell types. Such variation is necessary given the need for growth and differences in the function of various cell types. Due to its structural complexity, cell-cell variation and the paucity of analytical tools for complex carbohydrates, scientific progress toward understanding the cell wall has been relatively modest leaving large gaps in our knowledge. We are attempting to fill some of these gaps by using molecular genetics as an approach and the Arabidopsis seed coat as a model system.
The Arabidopsis seed coat has a specialized epidermis containing a large quantity of mucilage between a volcano shaped secondary cell wall and the primary cell wall. Upon exposure to water, the mucilage expands, rupturing the outer primary walls and encapsulating the seed to aid in dispersal, protection and/or hydration (Figure 1).

Figure 1. Arabidopsis seed with extruded mucilage stained with a red pectin specific dye.
Like primary cell walls, seed mucilage is composed of cellulose, hemicellulose, pectin and protein. When extruded, mucilage forms dark-staining rays perpendicular to the seed surface. The rays are adherent to the seed. Since mucilage has all the major components of the primary cell wall, is produced by a single cell type, is available in large quantities, has a distinct structure and is not required for seed viability, it is well suited as a genetic model for studying cell wall biogenesis, function and regulation.
Cellulose in extruded mucilage is a component of the ray that extends many cell lengths beyond the surface of the seed (Figure 2). Using molecular genetics we have investigated its function and how it is made and packaged in the seed coat epidermal cells (Mendu et al., 2011, Plant Physiol. 157: 441–453; Griffiths et al., 2015, Plant Physiol. 168: 502-520).

Figure 2. Arabidopsis seed with extruded mucilage stained with a red cellulose specific dye.
Mucilage matrix components (pectin, hemicellulose) are made in seed coat epidermal cells and secreted to the apoplast forming a mucilage pocket between the cell membrane and the primary cell wall (Figure 3).

Figure 3. Developing seed coat showing an epidermal cell with volcano shaped cytoplasm and a mucilage pocket (purple) between the cytoplasm and the outer primary cell wall.
Similar to cellulose synthesis in other cell walls, the cellulose in seed mucilage is made by cellulose synthase (CESA) complexes in the membrane. CESA3 and CESA5 are CESA subunit polypeptides that are part of the complex in seed coat epidermal cells and are found in the membrane lining the mucilage pocket (Figure 4).
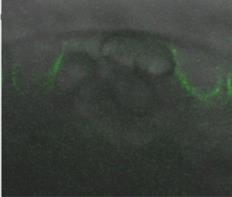
Figure 4. Seed coat epidermal cell showing fluorescent CESA5 in the membrane around the developing mucilage pocket.
These complexes move in a linear, unidirectional fashion around the cytoplasmic column of the cell, parallel with the surface of the seed presumably synthesizing cellulose that is coiled around the volcano shaped cytoplasmic column. This cellu-lose appears to unravel as the mucilage extrudes, forming a linear ray (Figure 5).

Figure 5. Diagram showing cellulose and pectin in unextruded and extruded mucilage of a seed coat epidermal cell.
Both the speed of extrusion and the adherence of the mucilage are dependent on this cellulose. The fact that normal pectin is also seems to be required for both processes (Griffiths et al., 2014, Plant Physiology, 165: 991-1004) suggests that these unique properties of mucilage require an interaction between the cellulose and pectin components of mucilage.






